
Chemistry, 30.03.2020 21:46 deanazilyiah
A major component of gasoline is octane . When octane is burned in air, it chemically reacts with oxygen gas to produce carbon dioxide and water . What mass of "oxygen gas is consumed" by the reaction of 6.1 g of octane

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
A major component of gasoline is octane . When octane is burned in air, it chemically reacts with ox...
Questions


Mathematics, 29.05.2020 18:03

Mathematics, 29.05.2020 18:03


Mathematics, 29.05.2020 18:03

History, 29.05.2020 18:57

Mathematics, 29.05.2020 18:57

English, 29.05.2020 18:57






Mathematics, 29.05.2020 18:57


Social Studies, 29.05.2020 18:57

Health, 29.05.2020 18:57

Mathematics, 29.05.2020 18:57


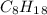 and its molecular mass is 114.23 g/mol.
and its molecular mass is 114.23 g/mol.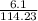 = 0.053 moles
= 0.053 moles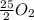 =
= 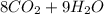
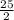 moles of oxygen.
moles of oxygen.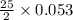 = 0.663 moles oxygen
= 0.663 moles oxygen


