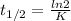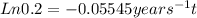Chemistry, 30.03.2020 21:33 erica11223344
The age of water or wine may be determined by measuring its radioactive tritium(3H) content. Tritium, present in a steady state in nature, is formed primarily by cosmicirradiation of water vapor in the upper atmosphere, and it decays spontaneously by a firstorder process with a half-life of 12.5 years. The formation reaction does not occursignificantly inside a glass bottle at the surface of the earth.
Calculate the age of a suspected vintage wine that is 20% as radioactive as a freshly bottled specimen. Radioactivity follows first order rate kinetics.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
The age of water or wine may be determined by measuring its radioactive tritium(3H) content. Tritium...
Questions



Social Studies, 10.03.2020 04:56




English, 10.03.2020 04:57


Mathematics, 10.03.2020 04:57





Mathematics, 10.03.2020 04:57


English, 10.03.2020 04:57




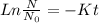 (1)
(1)