
Chemistry, 30.03.2020 21:22 dpazmembreno
A 35.0 L sample of gas collected in the upper atmosphere at a pressure of 48.6 torr is compressed into a 150. mL container at constant temperature. What is the new pressure (atm)? (4pts) 1 atm = 760 torr

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1


Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
A 35.0 L sample of gas collected in the upper atmosphere at a pressure of 48.6 torr is compressed in...
Questions

Chemistry, 13.04.2021 20:10




Mathematics, 13.04.2021 20:10


Mathematics, 13.04.2021 20:10

Mathematics, 13.04.2021 20:10



Social Studies, 13.04.2021 20:10


Mathematics, 13.04.2021 20:10

Physics, 13.04.2021 20:10


Mathematics, 13.04.2021 20:10

Mathematics, 13.04.2021 20:10

Mathematics, 13.04.2021 20:10

English, 13.04.2021 20:10

Mathematics, 13.04.2021 20:10

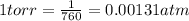
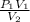
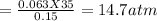 or 11172 torr
or 11172 torr 

