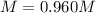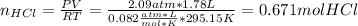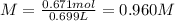Chemistry, 30.03.2020 21:05 jailenevazquez755
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 mL of water to form hydrochloric acid solution. Calculate the molarity of the solution. Assume no change in volume.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
A 1.78−L sample of hydrogen chloride (HCl) gas at 2.09 atm and 22°C is completely dissolved in 699 m...
Questions


History, 27.08.2019 18:40

Biology, 27.08.2019 18:40


Biology, 27.08.2019 18:40



History, 27.08.2019 18:40

History, 27.08.2019 18:40

Computers and Technology, 27.08.2019 18:40



Biology, 27.08.2019 18:40






Mathematics, 27.08.2019 18:40