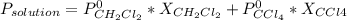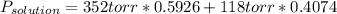Chemistry, 30.03.2020 21:07 villarrealc1987
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) among the many cancer causing volatile chlorinated organic compounds. An autoshop you are employed at keeps a jar (closed) of this solution to use as a solvent for cleaning parts. If the jar contains 1.60mol of dichloromethane and 1.10mol of CCl4, what would be the total pressure in the jar if the shop is kept at a consistent 23.5C

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

You know the right answer?
The US Food and Drug Administration lists dichloromethane (CH2Cl2) and carbon tetrachloride (CCl4) a...
Questions

Mathematics, 02.05.2021 22:20

Mathematics, 02.05.2021 22:20

Mathematics, 02.05.2021 22:20

Physics, 02.05.2021 22:20


History, 02.05.2021 22:20








Mathematics, 02.05.2021 22:20

Mathematics, 02.05.2021 22:20