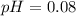Chemistry, 30.03.2020 21:10 Franklyn3220
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enough water to make a final volume of 75.0 mLmL. Part A Assuming that all of the solid dissolves, what is the pH of the final solution

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 08:00
Drag each pressure unit with the corresponding number to describe standard atmospheric pressure
Answers: 1

You know the right answer?
A solution is made by adding 0.380 gg Ca(OH)2(s)Ca(OH)2(s), 50.0 mLmL of 1.45 MM HNO3HNO3, and enoug...
Questions

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Arts, 15.12.2020 01:00



Mathematics, 15.12.2020 01:00


History, 15.12.2020 01:00



Biology, 15.12.2020 01:00


Mathematics, 15.12.2020 01:00

English, 15.12.2020 01:00

Mathematics, 15.12.2020 01:00

Advanced Placement (AP), 15.12.2020 01:00


Chemistry, 15.12.2020 01:00

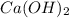 =
=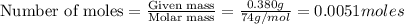
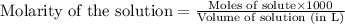
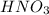 solution = 1.45 M
solution = 1.45 M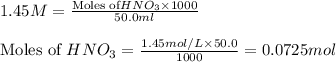
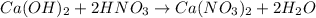
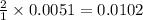 moles of
moles of 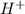 are left in 75.0 ml of solution
are left in 75.0 ml of solution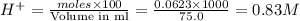
![pH=-\log [H^+]](/tpl/images/0571/2204/37e81.png)
![pH=-\log[0.83]](/tpl/images/0571/2204/f1115.png)