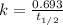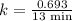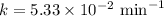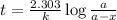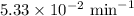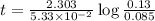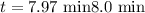Chemistry, 30.03.2020 20:27 smancilla2020
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes min for it to decrease to 0.085 M. A) 12 B) 10. C) 8.0 D) 11 E) 5.0 C

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

You know the right answer?
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13...
Questions



Mathematics, 22.07.2019 00:32

English, 22.07.2019 00:32


History, 22.07.2019 00:32



Social Studies, 22.07.2019 00:32

Mathematics, 22.07.2019 00:32



Mathematics, 22.07.2019 00:32


Mathematics, 22.07.2019 00:32


Mathematics, 22.07.2019 00:32


History, 22.07.2019 00:32

History, 22.07.2019 00:32