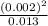Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
0.030 moles of a weak acid, HA, was dissolved in 2.0 L of water to form a solution. At equilibrium,...
Questions

History, 09.06.2021 21:50





Mathematics, 09.06.2021 21:50





Social Studies, 09.06.2021 21:50

Geography, 09.06.2021 21:50

Mathematics, 09.06.2021 22:00






Chemistry, 09.06.2021 22:00

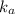 for the weak acid is
for the weak acid is 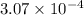 .
.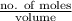
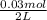
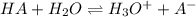
![k_{a} = \frac{[A^{-}][H_{3}O^{+}]}{[HA]}](/tpl/images/0571/0012/aea8e.png)
![\frac{x^{2}}{[HA]}](/tpl/images/0571/0012/57461.png)