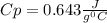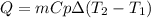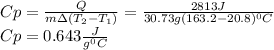Chemistry, 30.03.2020 22:18 Hockeypro1127
A 30.73 g 30.73 g sample of a substance is initially at 20.8 ° C. 20.8 °C. After absorbing 2813 J 2813 J of heat, the temperature of the substance is 163.2 ° C. 163.2 °C. What is the specific heat ( c c ) of the substance?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
A 30.73 g 30.73 g sample of a substance is initially at 20.8 ° C. 20.8 °C. After absorbing 2813 J 28...
Questions


History, 20.07.2019 02:30

English, 20.07.2019 02:30

English, 20.07.2019 02:30

Mathematics, 20.07.2019 02:30




English, 20.07.2019 02:30

History, 20.07.2019 02:30

Social Studies, 20.07.2019 02:30







Mathematics, 20.07.2019 02:30