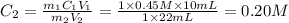The pH on the y scale is from 0-14 and the volume of titrant in mL is from 0-34. The equivalence point is around 22. The question is: "The above titration curve was obtained when a 10 mL sample of a 0.45 M base was titrated with an acid. What is the approximate molarity of the acid used?" (Assume a 1:1 molar ratio)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
The pH on the y scale is from 0-14 and the volume of titrant in mL is from 0-34. The equivalence poi...
Questions

English, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01



Social Studies, 23.09.2020 14:01


Spanish, 23.09.2020 14:01



History, 23.09.2020 14:01



Biology, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01



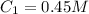
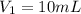
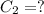
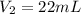
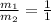
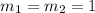
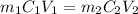 ( neutralization )
( neutralization )