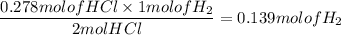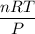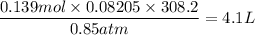Chemistry, 30.03.2020 19:29 dazesreplayy5363
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calculate the volume(in L) of H2(g) at 35.2°C and 646 torr that can be formed when 325 mL of 0.855 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
Mg(s) + HCl(aq) --> MgCl2(aq) + H2(g) (unbalanced)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved. Calcula...
Questions

Mathematics, 11.09.2021 04:50

Computers and Technology, 11.09.2021 04:50



Mathematics, 11.09.2021 04:50


Mathematics, 11.09.2021 04:50


Mathematics, 11.09.2021 04:50

Mathematics, 11.09.2021 04:50

Chemistry, 11.09.2021 04:50


Physics, 11.09.2021 04:50


Mathematics, 11.09.2021 04:50


Computers and Technology, 11.09.2021 04:50


Physics, 11.09.2021 04:50