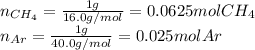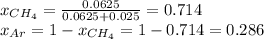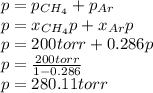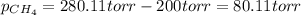Chemistry, 30.03.2020 19:32 bvbbridesmaid5519
A gas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0). If the partial pressure of argon is 200. torr, what is the pressure of methane, in torr

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
A gas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 4...
Questions



Health, 06.07.2019 03:30


Biology, 06.07.2019 03:30





Social Studies, 06.07.2019 03:30

Mathematics, 06.07.2019 03:30

Mathematics, 06.07.2019 03:30

Biology, 06.07.2019 03:30


Biology, 06.07.2019 03:30



Mathematics, 06.07.2019 03:30


English, 06.07.2019 03:30