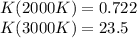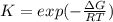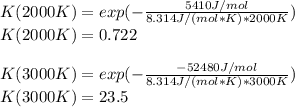The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X 2 ( g ) ⟶ X ( g ) Assume that the standard molar Gibbs energy of formation of X(g) is 5.41 kJ⋅mol − 1 at 2000. K and − 52.48 kJ⋅mol − 1 at 3000. K. Determine the value of K (the thermodynamic equilibrium constant) at each temperature. K at 2000. K = K at 3000. K =

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
The equation represents the decomposition of a generic diatomic element in its standard state. 1 2 X...
Questions






Mathematics, 16.03.2020 18:00









Computers and Technology, 16.03.2020 18:01


Mathematics, 16.03.2020 18:01

Mathematics, 16.03.2020 18:01

Business, 16.03.2020 18:01

Biology, 16.03.2020 18:01