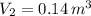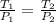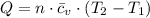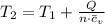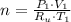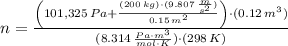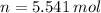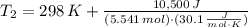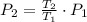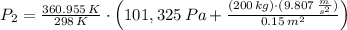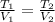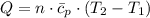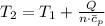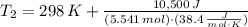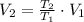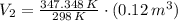Chemistry, 30.03.2020 19:18 minileiva211
A frictionless piston cylinder device is subjected to 1.013 bar external pressure. The piston mass is 200 kg, it has an area of 0.15 m2, and the initial volume of the entrapped ideal gas is 0.12 m3. The piston and cylinder do not conduct heat, but heat can be added to the gas by a heating coil. The gas has a constant-volume heat capacity of 30.1 J/(mol K) and an initial temperature of 298 K, and 10.5 kJ of energy are to be supplied to the gas through the heating coil.
a.) If stops placed at the initial equilibrium position of the piston prevent it from rising, what will be the final temperature and pressure of the gas?
b.) If the piston is allowed to move freely, what will be the final temperature and volume of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
A frictionless piston cylinder device is subjected to 1.013 bar external pressure. The piston mass i...
Questions

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Biology, 14.07.2020 02:01

Biology, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01





Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

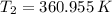 ,
, 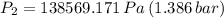 , b)
, b) 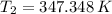 ,
,