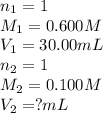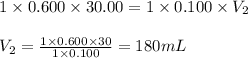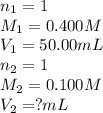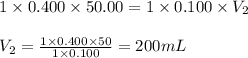Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
Determine the volume at the equivalence point if a 0.100 M NaOH(aq) solution is used to titrate the...
Questions


Mathematics, 13.05.2021 23:10


Mathematics, 13.05.2021 23:10

Social Studies, 13.05.2021 23:10



Mathematics, 13.05.2021 23:10


Mathematics, 13.05.2021 23:10



Computers and Technology, 13.05.2021 23:10





Biology, 13.05.2021 23:10

SAT, 13.05.2021 23:10

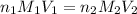
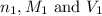 are the n-factor, molarity and volume of acid
are the n-factor, molarity and volume of acid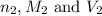 are the n-factor, molarity and volume of base
are the n-factor, molarity and volume of base