
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following reversible reaction occurs: CH3OH (g) D 2 H2 (g) +CO (g) At equilibrium, there were 3 moles of CH3OH in the container. What is the total number of moles of gas present in the container at equilibrium?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1
You know the right answer?
A sealed isothermal container initially contains 4 moles of methanol gas, CH3OH. The following rever...
Questions



Physics, 17.02.2022 19:20

Mathematics, 17.02.2022 19:20


English, 17.02.2022 19:20


SAT, 17.02.2022 19:20


Physics, 17.02.2022 19:20

Computers and Technology, 17.02.2022 19:20



Business, 17.02.2022 19:20

Social Studies, 17.02.2022 19:20



Mathematics, 17.02.2022 19:20


Mathematics, 17.02.2022 19:20

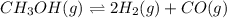
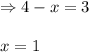
![[n_{CH_3OH}+n_{H_2}+n_{CO}]=[3+2+1]=6mol](/tpl/images/0570/7796/f27cd.png)


