

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
Consider the reaction: 4 NH3 + 7 O2 --> 4 NO2 + 6 H2O. At a certain instant the initial rate of d...
Questions




Mathematics, 11.08.2019 05:10

Mathematics, 11.08.2019 05:10






English, 11.08.2019 06:10

Social Studies, 11.08.2019 06:10



Mathematics, 11.08.2019 06:10



Mathematics, 11.08.2019 06:10


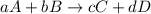
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0570/6613/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0570/6613/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0570/6613/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0570/6613/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0570/6613/d4b94.png)
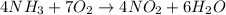
![\text{Rate of disappearance of }NH_3=-\frac{1}{4}\frac{d[NH_3]}{dt}](/tpl/images/0570/6613/523c5.png)
![\text{Rate of disappearance of }O_2=-\frac{1}{7}\frac{d[O_2]}{dt}](/tpl/images/0570/6613/a1c8e.png)
![\text{Rate of formation of }NO_2=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0570/6613/2ef8c.png)
![\text{Rate of formation of }H_2O=+\frac{1}{6}\frac{d[H_2O]}{dt}](/tpl/images/0570/6613/4e047.png)
![Rate=-\frac{1}{4}\frac{d[NH_3]}{dt}=-\frac{1}{7}\frac{d[O_2]}{dt}=+\frac{1}{4}\frac{d[NO_2]}{dt}=+\frac{1}{6}\frac{d[H_2O]}{dt}](/tpl/images/0570/6613/c1beb.png)
![-\frac{d[O_2]}{dt}=X](/tpl/images/0570/6613/a5858.png)
![-\frac{1}{7}\frac{d[O_2]}{dt}=+\frac{1}{6}\frac{d[H_2O]}{dt}](/tpl/images/0570/6613/4bb1d.png)
![\frac{d[H_2O]}{dt}=\frac{6}{7}\frac{d[O_2]}{dt}](/tpl/images/0570/6613/0a653.png)
![\frac{d[H_2O]}{dt}=\frac{6}{7}\times X](/tpl/images/0570/6613/ac1d5.png)
![\frac{d[H_2O]}{dt}=0.857X](/tpl/images/0570/6613/0ed54.png)



