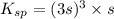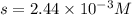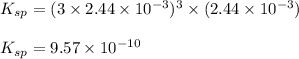Chemistry, 30.03.2020 17:56 gracie0818
The solubility of silver(I)phosphate at a given temperature is 1.02 g/L. Calculate the Ksp at this temperature. After you get your answer, take the negative log and enter that (so it's like you're taking the pKsp)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
The solubility of silver(I)phosphate at a given temperature is 1.02 g/L. Calculate the Ksp at this t...
Questions


Mathematics, 17.02.2021 19:30


English, 17.02.2021 19:30


History, 17.02.2021 19:30

Mathematics, 17.02.2021 19:30


History, 17.02.2021 19:30

Mathematics, 17.02.2021 19:30


Mathematics, 17.02.2021 19:30

Chemistry, 17.02.2021 19:30

Computers and Technology, 17.02.2021 19:30


Chemistry, 17.02.2021 19:30


Mathematics, 17.02.2021 19:30

Mathematics, 17.02.2021 19:30

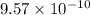
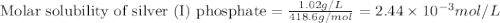
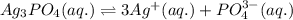
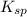 for above equation follows:
for above equation follows: