

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 01:30
Ascientist conducted an experiment and discovered that certain plants grow faster when given a particular amount of fertilizer. anouther scientist conducted the same experiment and got similar results. which concept does this best illustrate? a) repetition b) replication c) precision d) validity
Answers: 2
You know the right answer?
Suppose in an experiment to determine the amount of sodium hypochlorite in bleach, 0.0000524 mol K I...
Questions

Social Studies, 04.03.2021 22:40



English, 04.03.2021 22:40

Mathematics, 04.03.2021 22:40

Biology, 04.03.2021 22:40

Mathematics, 04.03.2021 22:40

Mathematics, 04.03.2021 22:40

Advanced Placement (AP), 04.03.2021 22:40



English, 04.03.2021 22:40



Mathematics, 04.03.2021 22:40

English, 04.03.2021 22:40




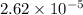 moles
moles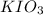 solution given = 0.0000524 moles
solution given = 0.0000524 moles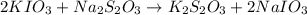
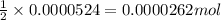 of sodium thiosulfate
of sodium thiosulfate

