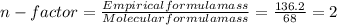A 5.769 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis and 14.92 grams of CO2 and 3.054 grams of H2O are produced. In a separate experiment, the molar mass is found to be 136.2 g/mol. Determine the empirical formula and the molecular formula of the organic compound.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1
You know the right answer?
A 5.769 gram sample of an organic compound containing C, H and O is analyzed by combustion analysis...
Questions

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

English, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Chemistry, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

Mathematics, 13.09.2020 21:01

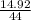 = 0.339 moles of carbonno. of moles of H₂O =
= 0.339 moles of carbonno. of moles of H₂O = 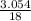 = 0.16 moles x 2 = 0.339 moles of hydrogenmass of carbon = 0.339 x 12 = 4.068 gmass of hydrogen = 0.339 x 1 = 0.339 gmass of oxygen = 5.769 - (4.068 + 0.339)
= 0.16 moles x 2 = 0.339 moles of hydrogenmass of carbon = 0.339 x 12 = 4.068 gmass of hydrogen = 0.339 x 1 = 0.339 gmass of oxygen = 5.769 - (4.068 + 0.339)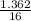 = 0.085 moles
= 0.085 moles 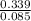 = 4relative mole ratio for hydrogen =
= 4relative mole ratio for hydrogen = 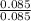 = 1 empirical formula C₄H₄Oempirical formula mass = 4 x 12 + 4 x 1 + 1 x 16 = 68molecular mass = 136.2 g / mol
= 1 empirical formula C₄H₄Oempirical formula mass = 4 x 12 + 4 x 1 + 1 x 16 = 68molecular mass = 136.2 g / mol