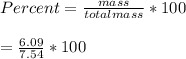Chemistry, 30.03.2020 17:42 haileesprague3999
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the reaction:
CaCO3(s) + 2 HCl (aq) → CaCl2 (aq) + CO2(g) + H2O (l)
When a 7.54 g piece of chalk is placed in HCl, 2.68 of CO2 is produced. What percentage of the chalk is CaCO3?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Based on the position vs. time graph which velocity vs. time graph would correspond to the data
Answers: 2

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 18:50
Question 3(multiple choice worth 4 points) (04.04 lc) what does it mean when an element is reduced? it empties a valance shell, reducing its atomic radius. it gains electrons, reducing its overall charge. it increases electronegativity, reducing its ability to bond. it loses electrons, reducing its electron number.
Answers: 1
You know the right answer?
Chalk is a mixture of CaCO3 and CaSO4. When added to HCl, only the CaCO3 reacts according to the rea...
Questions

Mathematics, 22.05.2020 19:01

Spanish, 22.05.2020 19:01


Mathematics, 22.05.2020 19:01


Mathematics, 22.05.2020 19:01

English, 22.05.2020 19:01


Mathematics, 22.05.2020 19:01



Computers and Technology, 22.05.2020 19:01

History, 22.05.2020 19:01


History, 22.05.2020 19:01




Mathematics, 22.05.2020 19:01

Computers and Technology, 22.05.2020 19:01