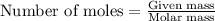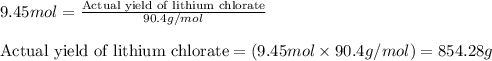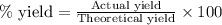Consider the balanced equation for the following reaction:
3Ca(ClO3)2(aq) + 2Li3PO4(aq) → Ca3(...

Chemistry, 30.03.2020 17:35 brazilmade1
Consider the balanced equation for the following reaction:
3Ca(ClO3)2(aq) + 2Li3PO4(aq) → Ca3(PO4)2(s) + 6LiClO3(aq)
Determine the theoretical yield of LiClO3(aq) in grams if the percent yield of LiClO3(aq) is 81.0% and 9.45 moles of LiClO3(aq) forms.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
Questions

English, 11.11.2020 23:40

English, 11.11.2020 23:40

Physics, 11.11.2020 23:40


SAT, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40

History, 11.11.2020 23:40



Social Studies, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40

Physics, 11.11.2020 23:40



Mathematics, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40


Mathematics, 11.11.2020 23:40

Mathematics, 11.11.2020 23:40