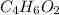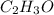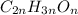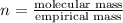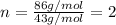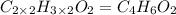Chemistry, 30.03.2020 17:30 legend101xD
Severus Snape explains that if you have the molar mass of a compound and the empirical formula of a compound you can determine the molecular formula of a compound. Suppose you have a compound which has an empirical formula of C2H3O and molar mass of 86 g/mol. What is the compounds molecular formula

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
Severus Snape explains that if you have the molar mass of a compound and the empirical formula of a...
Questions

Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00


Physics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00


Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00


History, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00

Chemistry, 21.04.2021 21:00

Mathematics, 21.04.2021 21:00


Computers and Technology, 21.04.2021 21:00

Social Studies, 21.04.2021 21:00