
Chemistry, 30.03.2020 16:55 jc95704816
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assume that all the radiation is absorbed and converted to heat. How many photons are required for the liquid to absorb 58.28 J 58.28 J of heat?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

You know the right answer?
A liquid is exposed to infrared radiation with a wavelength of 6.92 × 10 − 4 cm. 6.92×10−4 cm. Assum...
Questions


Mathematics, 20.04.2020 20:17


Mathematics, 20.04.2020 20:17



Mathematics, 20.04.2020 20:17

Arts, 20.04.2020 20:17


Social Studies, 20.04.2020 20:17

Mathematics, 20.04.2020 20:17

History, 20.04.2020 20:17


Mathematics, 20.04.2020 20:17






Mathematics, 20.04.2020 20:17

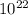 Photons
Photons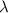 = 6.92 ×
= 6.92 × 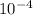 cm = 6.92 ×
cm = 6.92 × 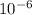 m
m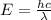
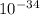 J - s
J - s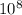
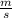
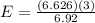 ×
× 
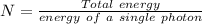
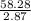 ×
× 

