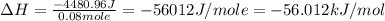Chemistry, 30.03.2020 17:02 Diamondnado3046
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperature of the solution goes from 25.10°C to 27.78° C. What is the molar enthalpy of neutralization of the acid in kJ/mol? Assume all densities are 1.00 g/mL and the specific heat capacities are 4.184 J/g*K.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Review the branily terms and services guides well u know what i never did so go have a nice ice cream sunday
Answers: 1

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2
You know the right answer?
You mix 200. mL of 0.400M HCl with 200. mL of 0.400M NaOH in a coffee cup calorimeter. The temperatu...
Questions

Mathematics, 30.08.2019 04:30

History, 30.08.2019 04:30

English, 30.08.2019 04:30

Social Studies, 30.08.2019 04:30


Computers and Technology, 30.08.2019 04:30


Mathematics, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Social Studies, 30.08.2019 04:30


History, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30



World Languages, 30.08.2019 04:30

Mathematics, 30.08.2019 04:30

Biology, 30.08.2019 04:30


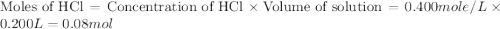
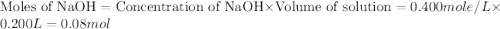

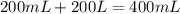
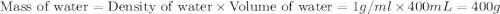
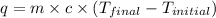
 = specific heat of water =
= specific heat of water = 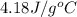
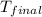 = final temperature of water =
= final temperature of water = 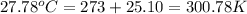
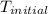 = initial temperature of metal =
= initial temperature of metal = 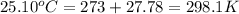
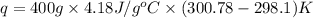
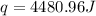
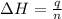
 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?