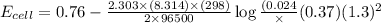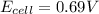In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+ half-cell and an H2/H+ half-cell under the following conditions: [Zn2+ ] = 0.024 M [H+ ]= 1.3 M partial pressure of H2 = 0.37 atm. Calculate Ecell at 298 K (enter to 3 decimal places). Zn2+ (aq) + 2eLaTeX: -− LaTeX: \longrightarrow⟶ Zn(s) E° = LaTeX: -−0.76 V 2H+ (aq) + 2eLaTeX: -−LaTeX: \longrightarrow⟶ H2(g) E° = 0.00 V

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
In a test of a new reference electrode, a chemist constructs a voltaic cell consisting of a Zn/Zn2+...
Questions




World Languages, 18.01.2021 06:10

Social Studies, 18.01.2021 06:10

Social Studies, 18.01.2021 06:10



Arts, 18.01.2021 06:10

Mathematics, 18.01.2021 06:10

Mathematics, 18.01.2021 06:10

Arts, 18.01.2021 06:10


Mathematics, 18.01.2021 06:10

Mathematics, 18.01.2021 06:10



Mathematics, 18.01.2021 06:10


Biology, 18.01.2021 06:10

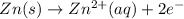
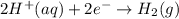
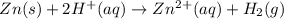
![E^o_{[Zn^{2+}/Zn]}=-0.76V](/tpl/images/0570/4548/c67da.png)
![E^o_{[H^+/H_2]}=0.00V](/tpl/images/0570/4548/4b48a.png)
![E^o=E^o_{[cathode]}-E^o_{[anode]}](/tpl/images/0570/4548/51f3e.png)
![E^o=E^o_{[H^+/H_2]}-E^o_{[Zn^{2+}/Zn]}](/tpl/images/0570/4548/91ffc.png)
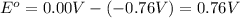
![E_{cell}=E^o_{cell}-\frac{2.303RT}{nF}\log \frac{[Zn^{2+}]\times (p_{H_2})}{[H^+]^2}](/tpl/images/0570/4548/307e3.png)
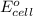 = electrode potential of the cell = ?
= electrode potential of the cell = ?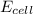 = emf of the cell = 0.76 V
= emf of the cell = 0.76 V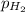 = 0.37 atm
= 0.37 atm![[Zn^{2+}]](/tpl/images/0570/4548/9c01a.png) = 0.024 M
= 0.024 M![[H^{+}]](/tpl/images/0570/4548/85507.png) = 1.3 M
= 1.3 M