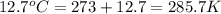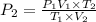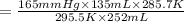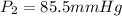Chemistry, 30.03.2020 16:52 haelleydorethy
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135 mL, and the second container, B, has a volume of 117 mL. A sample of gas originally in container A is at 22.5 C and the pressure is 165 mmHg. What is the pressure (in mmHg) of the gas sample when the valve is opened and the gas now occupies both containers at a temperature of 12.7 C

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
Two gas-containers, A and B, are connected with a valve. The first container, A, has a volume of 135...
Questions


History, 22.06.2019 16:00



Mathematics, 22.06.2019 16:00


Biology, 22.06.2019 16:00






History, 22.06.2019 16:00



Social Studies, 22.06.2019 16:00

History, 22.06.2019 16:00

Chemistry, 22.06.2019 16:00

Physics, 22.06.2019 16:00

History, 22.06.2019 16:00

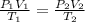
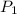 = initial pressure of gas in container A = 165 mmHg
= initial pressure of gas in container A = 165 mmHg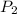 = final pressure of gas = ?
= final pressure of gas = ?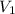 = initial volume of gas in container A=
= initial volume of gas in container A= 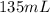
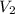 = final volume of gas = 135 mL + 117 mL = 252 mL
= final volume of gas = 135 mL + 117 mL = 252 mL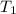 = initial temperature of gas in container A =
= initial temperature of gas in container A = 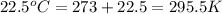
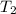 = final temperature of gas =
= final temperature of gas =