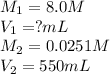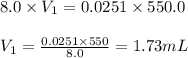A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He wi...

A chemist must prepare 550 mL of hydrochloric acid solution with a pH of 1.60 at 25°C .
He will do this in three steps:
-Fill a 550.0 mL volumetric flask about halfway with distilled water.
-Measure out a small volume of concentrated (8.0M) stock hydrochloric acid solution and add it to the flask.
-Fill the flask to the mark with distilled water.
Calculate the volume of concentrated hydrochloric acid that the chemist must measure out in the second step. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2
You know the right answer?
Questions

Chemistry, 04.03.2021 23:40




History, 04.03.2021 23:40






English, 04.03.2021 23:40


English, 04.03.2021 23:40






Mathematics, 04.03.2021 23:40

![pH=-\log[H^+]](/tpl/images/0570/3526/cf945.png)
![1.60=-\log [H^+]](/tpl/images/0570/3526/bdcbd.png)
![[H^+]=10^{-1.60}=0.0251M](/tpl/images/0570/3526/4bcd7.png)
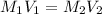
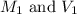 are the molarity and volume of the concentrated HCl solution
are the molarity and volume of the concentrated HCl solution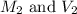 are the molarity and volume of diluted HCl solution
are the molarity and volume of diluted HCl solution