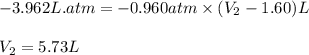Chemistry, 30.03.2020 04:55 ianball025
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable piston. Initially the cylinder contains a volume of 1.60 L. The sample is ignited producing gas and releasing 401.5 J of energy. To what volume will the cylinder expand to if it must expand against a pressure of 729.8 mmHg. Assume all the energy released is converted to work used to push the piston?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
A sample of flammable liquid is placed into an enclosed cylinder which is then fitted with a movable...
Questions

English, 20.12.2020 08:00


Mathematics, 20.12.2020 08:00

English, 20.12.2020 08:00


English, 20.12.2020 08:00

Business, 20.12.2020 08:00

Chemistry, 20.12.2020 08:00




Chemistry, 20.12.2020 08:00

English, 20.12.2020 08:00


Mathematics, 20.12.2020 08:10

Mathematics, 20.12.2020 08:10


History, 20.12.2020 08:10

English, 20.12.2020 08:10

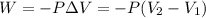
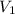 = initial volume = 1.60 L
= initial volume = 1.60 L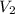 = final volume = ?
= final volume = ?