Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2
You know the right answer?
What volume of methanol should be used to make 1.25 L of a 21.0 % by volume solution?...
Questions

Geography, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00

Health, 13.02.2021 07:00


Social Studies, 13.02.2021 07:00

Mathematics, 13.02.2021 07:00



Spanish, 13.02.2021 07:00


Health, 13.02.2021 07:00


English, 13.02.2021 07:00


Mathematics, 13.02.2021 07:00


Mathematics, 13.02.2021 07:00

History, 13.02.2021 07:00


Computers and Technology, 13.02.2021 07:00

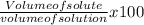
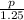 x 100
x 100