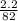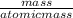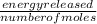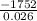A calorimeter contains 30.0 mL of water at 12.5 ∘C . When 2.20 g of X (a substance with a molar mass of 82.0 g/mol ) is added, it dissolves via the reaction X(s)+H2O(l)→X(aq) and the temperature of the solution increases to 25.5 ∘C . Calculate the enthalpy change, ΔH, for this reaction per mole of X. Assume that the specific heat of the resulting solution is equal to that of water [4.18 J/(g⋅∘C)], that density of water is 1.00 g/mL, and that no heat is lost to the calorimeter itself, nor to the surroundings

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 1

You know the right answer?
A calorimeter contains 30.0 mL of water at 12.5 ∘C . When 2.20 g of X (a substance with a molar mass...
Questions

Chemistry, 14.12.2021 07:00


Mathematics, 14.12.2021 07:00

Mathematics, 14.12.2021 07:00

English, 14.12.2021 07:00


English, 14.12.2021 07:00

Mathematics, 14.12.2021 07:00



Mathematics, 14.12.2021 07:00

Computers and Technology, 14.12.2021 07:00


World Languages, 14.12.2021 07:00


Biology, 14.12.2021 07:00

Mathematics, 14.12.2021 07:00


Mathematics, 14.12.2021 07:00

Mathematics, 14.12.2021 07:00