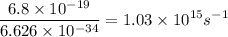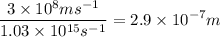Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
An energy of 6.8 x 10^-19 J/atom is required to cause an aluminum atom on a metal surface to lose an...
Questions

Mathematics, 22.11.2019 20:31


Mathematics, 22.11.2019 20:31

History, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31


Mathematics, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31

Computers and Technology, 22.11.2019 20:31


Mathematics, 22.11.2019 20:31

Mathematics, 22.11.2019 20:31

English, 22.11.2019 20:31

English, 22.11.2019 20:31


History, 22.11.2019 20:31


Mathematics, 22.11.2019 20:31

English, 22.11.2019 20:31

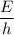 =
=