
How can an unknown A Heaction be determined using Hess's law?
A. The free energy of the reaction is used to determine the A H for
the reaction.
O
B. The reaction is repeated at different temperatures to determine
the A Hreaction
O
C. Enthalpies from reaction steps are added to determine an
unknown A Hreaction:
D. The unknown A Heaction is determined after the reaction is run in a
calorimeter.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

You know the right answer?
How can an unknown A Heaction be determined using Hess's law?
A. The free energy of the reacti...
A. The free energy of the reacti...
Questions



Mathematics, 20.11.2020 21:20




Mathematics, 20.11.2020 21:20

History, 20.11.2020 21:20

Chemistry, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20



Arts, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20


Mathematics, 20.11.2020 21:20

Mathematics, 20.11.2020 21:20

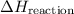 from reaction steps are combined to determine an unknown
from reaction steps are combined to determine an unknown 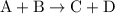 .
. 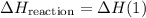 .Reaction (2):
.Reaction (2): 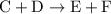 .
. 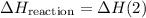 .
.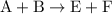 .
.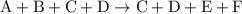 .
. and
and 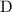 to get:
to get: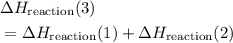 .
. is involved in this calculation. There's not even the need to carry out an experiment or take any new measurements. Because of that, Hess's Law can be very useful for finding the
is involved in this calculation. There's not even the need to carry out an experiment or take any new measurements. Because of that, Hess's Law can be very useful for finding the 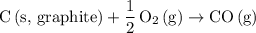 .
.

