Note the average atomic mass of copper on the nano-balance. Add atoms to the
larger balance un...

Note the average atomic mass of copper on the nano-balance. Add atoms to the
larger balance until it registers the same number in g) as the reading on the nano-balance
(in u). Use the Exponent slider to help get the correct amount. Stop adding atoms when the
readings on both balances matchi exactly to the nearest 0.001 y).
How many atoms did you need to add?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Questions

Mathematics, 30.06.2019 04:00


Mathematics, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00


History, 30.06.2019 04:00



Mathematics, 30.06.2019 04:00





History, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00


Social Studies, 30.06.2019 04:00


Social Studies, 30.06.2019 04:00

Mathematics, 30.06.2019 04:00

 1
1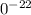 g
.
g
.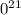 atoms.
atoms.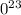 atoms or molecules of that substance - this is known as Avogadro's number.
atoms or molecules of that substance - this is known as Avogadro's number.

