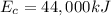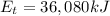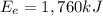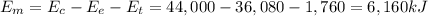Gasoline containing a total of 44,000 kJ of energy was burned in a car
engine. The table below...

Chemistry, 28.03.2020 21:15 daniellaZemira
Gasoline containing a total of 44,000 kJ of energy was burned in a car
engine. The table below shows some information related to the energy
output of the car from burning the gasoline. How much potential energy
from the gasoline was converted to mechanical energy? Energy Output
in (kJ) Thermal = 36,080 Electrical = 1,760 Mechanical = ?? *
6,160 kJ
14,960 kJ
0
20,240 kJ
44,000 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1


Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
Questions

Mathematics, 24.03.2021 20:40




Mathematics, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40



Mathematics, 24.03.2021 20:40

Biology, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40






English, 24.03.2021 20:40

Mathematics, 24.03.2021 20:40

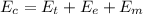
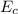 is the chemical energy contained in the gasoline (the energy in input)
is the chemical energy contained in the gasoline (the energy in input)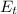 is the thermal energy in output
is the thermal energy in output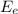 is the electrical energy in output
is the electrical energy in output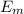 is the mechanical energy in output
is the mechanical energy in output