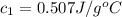Chemistry, 28.03.2020 17:59 meiyrarodriguez
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly insulated calorimeter. The final temperature of the metal-water mixture was 28.2ºC. Calculate the specific heat capacity of the metal using the data. Type your work and answer below. Make sure to include a unit on the final answer.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

You know the right answer?
A 24.87 gram sample of a metal at 104.0ºC was added to 76.12 grams of water at 25.2ºC in a perfectly...
Questions

Mathematics, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10

Mathematics, 30.10.2020 18:10





English, 30.10.2020 18:10

English, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10

English, 30.10.2020 18:10

Arts, 30.10.2020 18:10


Biology, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10


Physics, 30.10.2020 18:10

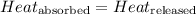
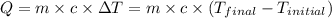
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0568/9675/09236.png) ......(1)
......(1)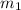 = mass of metal = 24.87 g
= mass of metal = 24.87 g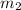 = mass of water = 76.12 g
= mass of water = 76.12 g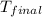 = final temperature = 28.2°C
= final temperature = 28.2°C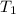 = initial temperature of metal = 104.0°C
= initial temperature of metal = 104.0°C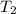 = initial temperature of water = 25.2°C
= initial temperature of water = 25.2°C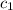 = specific heat of metal = ?
= specific heat of metal = ?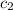 = specific heat of water = 4.186 J/g°C
= specific heat of water = 4.186 J/g°C![24.87\times c_1\times (28.2-104)=-[76.12\times 4.186\times (28.2-25.2)]](/tpl/images/0568/9675/a6f4b.png)