
Chemistry, 28.03.2020 05:51 aricketts3901
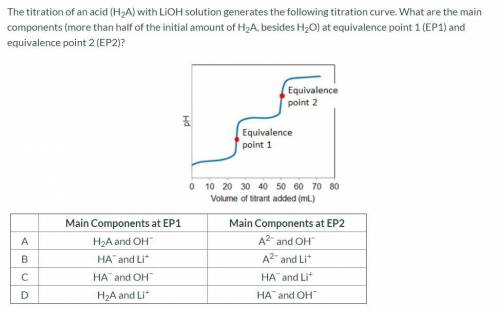
The titration of an acid (H2A) with LiOH solution generates the following titration curve. What are the main components (more than half of the initial amount of H2A, besides H2O) at equivalence point 1 (EP1) and equivalence point 2 (EP2)?
Main Components at EP1 Main Components at EP2
A H2A and OH− A2− and OH−
B HA− and Li+ A2− and Li+
C HA− and OH− HA− and Li+
D H2A and Li+ HA− and OH−

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2


Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
The titration of an acid (H2A) with LiOH solution generates the following titration curve. What are...
Questions

Mathematics, 02.03.2022 19:10


Mathematics, 02.03.2022 19:10


Social Studies, 02.03.2022 19:10



Mathematics, 02.03.2022 19:10



Physics, 02.03.2022 19:10

Health, 02.03.2022 19:10

Mathematics, 02.03.2022 19:10



English, 02.03.2022 19:20

Mathematics, 02.03.2022 19:20





