Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
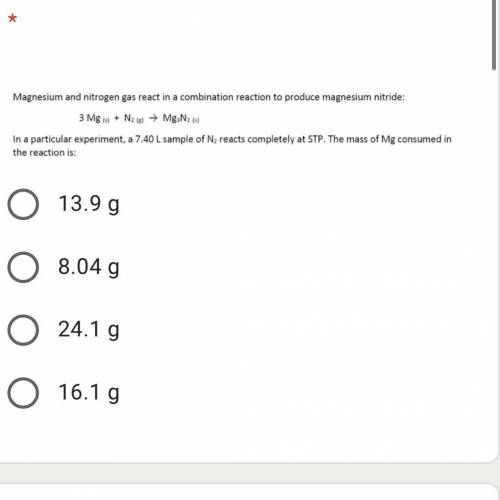
A 7.40 L sample of N2 reacts completely at STP. the mass of the Mg consumed in the reaction is:
Questions

Mathematics, 18.10.2021 19:50


Mathematics, 18.10.2021 19:50

Mathematics, 18.10.2021 19:50




English, 18.10.2021 19:50


Mathematics, 18.10.2021 19:50

Physics, 18.10.2021 19:50

Mathematics, 18.10.2021 19:50

Medicine, 18.10.2021 19:50

History, 18.10.2021 19:50


Mathematics, 18.10.2021 19:50

Mathematics, 18.10.2021 19:50

SAT, 18.10.2021 19:50

English, 18.10.2021 19:50

Social Studies, 18.10.2021 19:50