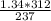Chemistry, 27.03.2020 22:28 LaughingAlanna
A gas has a pressure of 1.34 atm when the temperature is 237K. The gas is then heated until the temperature measures 312K. What will be the new pressure?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
A gas has a pressure of 1.34 atm when the temperature is 237K. The gas is then heated until the temp...
Questions

Mathematics, 18.11.2020 04:00

English, 18.11.2020 04:00


English, 18.11.2020 04:00






Geography, 18.11.2020 04:00

Business, 18.11.2020 04:00

Mathematics, 18.11.2020 04:00


Advanced Placement (AP), 18.11.2020 04:00

Mathematics, 18.11.2020 04:00

Mathematics, 18.11.2020 04:00


Mathematics, 18.11.2020 04:00

Mathematics, 18.11.2020 04:00

History, 18.11.2020 04:00

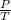 = constant
= constant 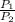 =
= 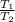
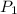 = initial pressure of a gas
= initial pressure of a gas 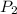 = final pressure of a gas
= final pressure of a gas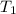 = initial temperature of a gas
= initial temperature of a gas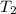 = final temperature of a gas
= final temperature of a gas