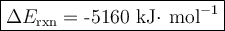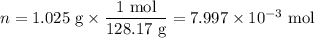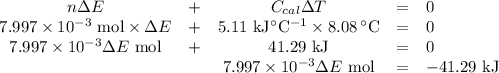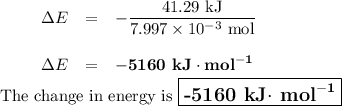Chemistry, 27.03.2020 21:01 usagimiller
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene is burned in a bomb calorimeter, the temperature rises from 24.25 ∘C to 32.33 ∘C. Find ΔErxn for the combustion of naphthalene. The heat capacity of the calorimeter, determined in a separate experiment, is 5.11kJ/∘C. Express the change in energy in kilojoules per mole to three significant figures.ΔErxn = kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
Mothballs are composed primarily of the hydrocarbon naphthalene (C10H8). When 1.025 gof naphthalene...
Questions


Chemistry, 27.08.2019 18:00

Physics, 27.08.2019 18:00

History, 27.08.2019 18:00




Chemistry, 27.08.2019 18:00


English, 27.08.2019 18:00

History, 27.08.2019 18:00




Mathematics, 27.08.2019 18:00

Mathematics, 27.08.2019 18:00

Biology, 27.08.2019 18:00


History, 27.08.2019 18:00

English, 27.08.2019 18:00