
Chemistry, 27.03.2020 20:59 jadenpittman02
2 _ Pb(SO )2 - LINO: –> Pb(NO3)4 + Li2SO4
li a student mixes 14 moles of C4H10O with 15 moles of O2: how many moles of Co2 can be
made?
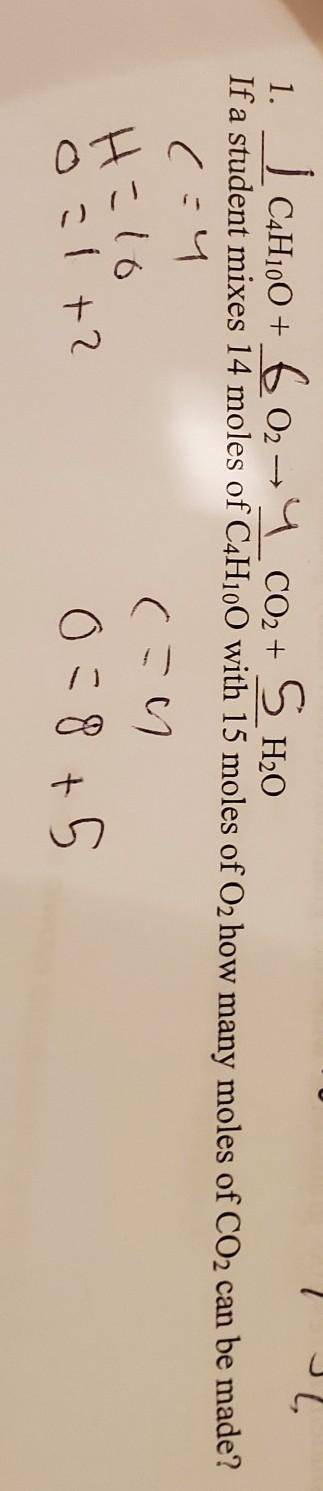

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
2 _ Pb(SO )2 - LINO: –> Pb(NO3)4 + Li2SO4
li a student mixes 14 moles of C4H10O with 15 mole...
li a student mixes 14 moles of C4H10O with 15 mole...
Questions

Chemistry, 14.07.2021 14:00



Computers and Technology, 14.07.2021 14:00

Health, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00



English, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00

Arts, 14.07.2021 14:00



Computers and Technology, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00

History, 14.07.2021 14:00


Social Studies, 14.07.2021 14:00

English, 14.07.2021 14:00



