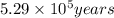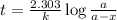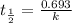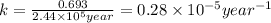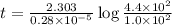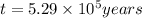Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If there are 4.4 x 102 g of the isotope in a small atomic bomb, how long will it take (in yr) for the substance to decay to 1.0 x 102 g, too small an amount for an effective bomb? This radioactive decay follows first order kinetics.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
Radioactive plutonium-239 (t½ = 2.44 x 105 yr) is used in nuclear reactors and atomic bombs. If ther...
Questions



Mathematics, 05.01.2020 16:31



World Languages, 05.01.2020 16:31

Social Studies, 05.01.2020 16:31

Chemistry, 05.01.2020 16:31

Arts, 05.01.2020 16:31




Physics, 05.01.2020 16:31



Mathematics, 05.01.2020 16:31

Mathematics, 05.01.2020 16:31


Mathematics, 05.01.2020 16:31

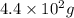 to
to  is
is