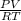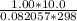Chemistry, 27.03.2020 18:26 ebookhardt917
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm. The volume of the helium is reduced to 10.4L, but the temperature and pressure of the gas are kept con- stant. What is the new quantity of the gas, in moles? Show all work using units and sig figs.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
A 4.00g sample of helium has a volume of 24.4L at a temperature of 25.0oC and a pressure of 1.00 atm...
Questions

Social Studies, 25.09.2019 20:30





English, 25.09.2019 20:30

English, 25.09.2019 20:30


Mathematics, 25.09.2019 20:30


Health, 25.09.2019 20:30

Physics, 25.09.2019 20:30


Biology, 25.09.2019 20:30


Mathematics, 25.09.2019 20:30

History, 25.09.2019 20:30

Biology, 25.09.2019 20:30

Business, 25.09.2019 20:30