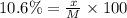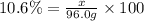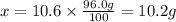Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
You know the right answer?
What mass of KNO3 is present in 96.0 g of a solution that is 10.6% KNO3 by mass?...
Questions

Chemistry, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00



Biology, 22.03.2021 01:00



History, 22.03.2021 01:00


Mathematics, 22.03.2021 01:00



Computers and Technology, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00

Mathematics, 22.03.2021 01:00


Computers and Technology, 22.03.2021 01:00