Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
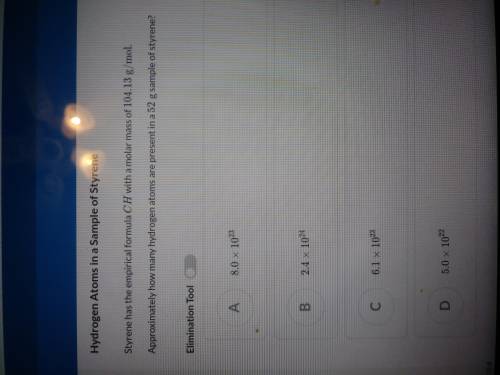
Styrene has an empirical formula CH with a molar mass of 104.13g/mol
Approximately how many hy...
Approximately how many hy...
Questions

Mathematics, 23.05.2021 16:30

Business, 23.05.2021 16:30

English, 23.05.2021 16:30


Mathematics, 23.05.2021 16:30




Mathematics, 23.05.2021 16:30




Mathematics, 23.05.2021 16:30

Social Studies, 23.05.2021 16:30


Biology, 23.05.2021 16:30



English, 23.05.2021 16:30