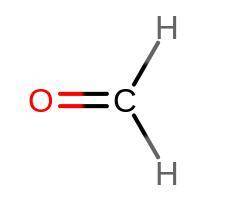
Identify the hybrid orbitals on all atoms in the molecule CH2O.
I don't understand how C...

Chemistry, 27.03.2020 00:47 mackenziesue8324
Identify the hybrid orbitals on all atoms in the molecule CH2O.
I don't understand how C and O wouldn't be 1 sp^2 if they're both trigonal planar's



Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 07:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1
You know the right answer?
Questions

Mathematics, 28.02.2021 06:40




Mathematics, 28.02.2021 06:40

English, 28.02.2021 06:40


English, 28.02.2021 06:40

Mathematics, 28.02.2021 06:40


Biology, 28.02.2021 06:40



Mathematics, 28.02.2021 06:40

Chemistry, 28.02.2021 06:40


Physics, 28.02.2021 06:40

Mathematics, 28.02.2021 06:40




