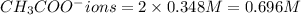In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to a total volume of 250 mL. What is the molarity of the solution? M. What is the concentration of the chromium(II) cation? M. What is the concentration of the acetate anion? M. Submit AnswerRetry Entire Group

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
In the laboratory you dissolve 14.8 g of chromium(II) acetate in a volumetric flask and add water to...
Questions

History, 24.09.2020 14:01

Physics, 24.09.2020 14:01


Advanced Placement (AP), 24.09.2020 14:01



History, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01


Spanish, 24.09.2020 14:01


Mathematics, 24.09.2020 14:01

Mathematics, 24.09.2020 14:01



Mathematics, 24.09.2020 14:01

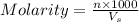
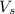 = volume of solution in ml = 150 ml
= volume of solution in ml = 150 ml
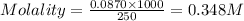
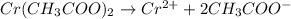
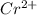 ions = 0.348 M
ions = 0.348 M