
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution at 22°C. Which statement explains why the contents of the flask are at equilibrium?The rate of dissolving is equal to the rate of crystallization. The rate of dissolving is greater than the rate of crystallization. The concentration of the solid is equal to the concentration of the solution. The concentration of the solid is greater than the concentration of the solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
Some solid KNO3 remains at the bottom of a stoppered flask containing a saturated KNO3(aq) solution...
Questions





Mathematics, 11.12.2020 01:10

Arts, 11.12.2020 01:10


Social Studies, 11.12.2020 01:10

Mathematics, 11.12.2020 01:10

Advanced Placement (AP), 11.12.2020 01:10

Social Studies, 11.12.2020 01:10

Mathematics, 11.12.2020 01:10

Social Studies, 11.12.2020 01:10

English, 11.12.2020 01:10


Mathematics, 11.12.2020 01:10

Mathematics, 11.12.2020 01:10


Arts, 11.12.2020 01:10

Mathematics, 11.12.2020 01:10

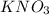 from aqueous
from aqueous 

