
Chemistry, 26.03.2020 22:42 elijahsantiago21
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 mL of a CH3COOH (acetic acid)/ CH3COONa * 3H2O buffer. The target pH of the buffer is 5.25. The given concentration of [CH3COOH] is equal to 0.10 M. Ka = 1.80 x 10-5 for acetic acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 m...
Questions

Mathematics, 07.10.2020 08:01

History, 07.10.2020 08:01

Mathematics, 07.10.2020 08:01





Mathematics, 07.10.2020 08:01




Mathematics, 07.10.2020 08:01


Mathematics, 07.10.2020 08:01


History, 07.10.2020 08:01



History, 07.10.2020 08:01

Social Studies, 07.10.2020 08:01

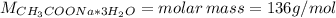
![pH = pKa + log(\frac{[CH_{3}COONa*3H_{2}O]}{[CH_{3}COOH]})](/tpl/images/0566/0820/ec252.png) (1)
(1)![log [CH_{3}COONa*3H_{2}O] = pH - pKa + log [CH_{3}COOH]](/tpl/images/0566/0820/d2774.png)
![log [CH_{3}COONa*3H_{2}O] = 5.25 - (-log(1.80 \cdot 10^{-5})) + log (0.10) = -0.495](/tpl/images/0566/0820/9589c.png)
![[CH_{3}COONa*3H_{2}O] = 10^{-0.495} = 0.32 M](/tpl/images/0566/0820/c6ecf.png)
![m = moles*M = [CH_{3}COONa*3H_{2}O]*V*M = 0.32 mol/L*0.250 L*136 g/mol = 10.88 g](/tpl/images/0566/0820/427f5.png)


