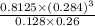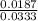Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1


Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous CH4 and H2...
Questions



Mathematics, 12.11.2020 21:50

Chemistry, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

Social Studies, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50



History, 12.11.2020 21:50


Mathematics, 12.11.2020 21:50

History, 12.11.2020 21:50


Social Studies, 12.11.2020 21:50

Mathematics, 12.11.2020 21:50

History, 12.11.2020 21:50

![[H_{2}O]](/tpl/images/0566/0391/04475.png) at equilibrium is 0.561 M.
at equilibrium is 0.561 M.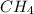 = 0.041 mol,
= 0.041 mol,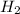 = 0.091 mol
= 0.091 mol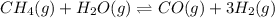
![\frac{[CO][H_{2}]^{3}}{[CH_{4}][H_{2}O]}](/tpl/images/0566/0391/7f129.png) ...... (1)
...... (1)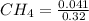 = 0.128 M,
= 0.128 M,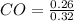 = 0.8125 M,
= 0.8125 M, 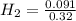 = 0.284 M
= 0.284 M![[H_{2}O] = \frac{[CO][H_{2}]^{3}}{[CH_{4}] \times K}](/tpl/images/0566/0391/7448a.png)